The direct methanol fuel cell (DMFC) is a power generation device that directly converts the chemical energy of the methanol oxidation reaction into electrical energy. Its working principle is very simple, and is mainly composed of a cathode, an anode, a proton exchange membrane, and a bipolar plate. During operation, methanol is catalytically oxidized to CO2 and H2O at the anode, and produces 6 electrons and 6 protons. Protons pass from the anode to the cathode through the proton exchange membrane, and the oxygen in the cathode compartment is reduced by the catalyst to generate H2O. The electrons reach the cathode from the anode through the external circuit and work through the external circuit to form an electrical circuit. The DMFC has a simple structure, is convenient and flexible, and the working time only depends on the amount of fuel carried and is not limited by the rated capacity of the battery. In recent years, the DMFC has been favored by the industry. DMFC does not need to go through Carnot cycle in the process of power generation. It has the advantages of high energy conversion efficiency, low emission and no noise. It also has the advantages of room temperature use, convenient fuel carrying recharge, high energy density of volume and weight ratio, and is particularly suitable for Small portable and portable power supplies have potential applications in defense, energy, and communications. At present, one of the major obstacles to the commercialization of DMFCs is the "methanol infiltration problem." This is because Nafion series perfluorinated sulfonic acid type proton exchange membranes commonly used in DMFC have a high methanol permeability. Methanol can pass through the proton exchange membrane from the anode to the cathode, and since the cathode generally uses Pt as a catalyst, oxygen Reduction and methanol oxidation occur at the same time, thus creating a "mixing potential" that severely reduces fuel efficiency and battery output. Moreover, the oxidation of methanol at the cathode also reduces its utilization. In addition, the diffused methanol and its oxidized intermediates also poison the cathode Pt catalyst, affecting the catalytic activity of Pt for oxygen reduction. Due to the presence of methanol permeation, DMFC generally maintains the fuel methanol concentration below 4 M. However, to compete with mainstream lithium-ion batteries currently on the market, the concentration of methanol used in DMFC must be increased to more than 9 M to effectively increase the energy density of the battery. The traditional strategies to overcome the methanol infiltration in DMFC include improving the fuel feeding system, enhancing the performance of proton membranes, modifying the structure of the battery electrode, and increasing the water management system. These strategies have indeed improved the operating performance of the battery to a certain degree, but undoubtedly the performance of the battery. The design tends to be complicated and increases the cost of battery manufacturing. Researcher Yang Jun, researcher of the National Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, switched research ideas to overcome the problem of methanol permeation in DMFC from the viewpoint of preparation of selective catalysts in order to reduce or get rid of proton membranes. Dependency. The selective catalyst means that the catalytic material used at the cathode or anode of the DMFC only catalyzes the reaction of the cathode or the anode and has no activity or very low reactivity to the reaction of the other side. Based on a deep understanding of the mechanism of methanol catalytic oxidation and oxygen catalytic reduction in DMFCs, they designed precious metal-based heterostructure nanomaterials to make full use of lattice strain effects and electronic coupling effects in heterogeneous materials to regulate the catalytic properties of materials, not only The material has excellent catalytic activity, and makes the material have good selectivity for methanol oxidation or oxygen reduction in DMFC. Specifically, they use a ternary nanocomposite Au@Ag2S@Pt with a core-shell-shell structure and Au@Pd nanomaterials with a core-shell structure as the anode and cathode electrocatalysts of the DMFC, respectively. For the former, the electronic coupling effect in the ternary material increases the density of the electron cloud of the Pt atom, suppresses the adsorption of carbon monoxide (CO) and oxygen (O2) molecules on the Pt atom, making it have excellent methanol oxidation activity while having Weak redox activity; and for the latter, due to the difference in lattice parameters and electronegativity, the lattice stretching effect and electron coupling effect of the Au core applied to the ultra-thin Pd shell enhances the Pd-catalyzed oxygen well. The activity of the reduction, and because Pd is not active in the oxidation of methanol in acidic media, makes this core-shell structural material a suitable candidate for the cathodic selectivity catalyst for DMFC. The researchers studied the preparation, amplification, and characterization of the catalysts and successfully assembled a DMFC cell on the basis of the demonstration of catalyst selectivity using a proton-free membrane DMFC model (pictured). Tests have shown that at a methanol concentration of 10 M, the power density of the battery output is 89.7 mW/cm2, which is much higher than the output power density of the DMFC reported using other strategies to achieve high concentration of methanol operation in recent years. When the methanol concentration is increased to 15 M, the output power of the battery is slightly reduced, and the power density output of 82.7 mW/cm2 can still be maintained. The above related research has been supported by the National Natural Science Foundation of China (No. 21376247, 21506225, 21573240) and the Institute of Process Engineering of the Chinese Academy of Sciences (COM2015A001). The study was published in the "Science Advances, 2017, 3: e1700580" international journal published by the American Association for the Advancement of Science (AAAS). Generally, they can only kill mites, but they can't kill insects and have more varieties, but their main activity is to kill insects, not to be known as acaricide, and sometimes they are also called insecticidal and acaricide. A class of pesticides used to harm the pests of various plants, stores, livestock and other arachnids. Among the acaricides, some species have high activity of active mite (mites and mites, weak mites), poor egg activity, and even invalid. Some species have high egg activity, bad effect on or dynamic mite; Some varieties can be killed. The common species of acaricide are bromoesters, avidin, spirofenesters, azoesters. Agrochemical Insecticide&Acaricide Agrochemical Insecticide,Agrochemical Acaricide,Agrochemical Fungicide,Agrochemical Raw Material Insecticide Hebei Senton International Trading Co.,Ltd. , https://www.sentonpharm.com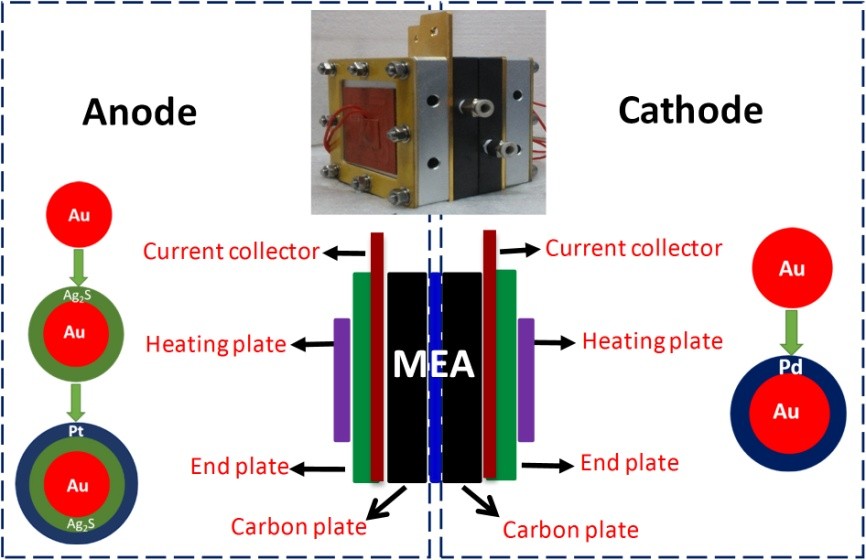
Diagram of Direct Methanol Fuel Cell and Its Components Based on Selective Electrocatalyst Assembly