According to foreign media reports, metal-air batteries have been regarded as the "heirs" of lithium-ion batteries because of their excellent weight energy density. Metal-air batteries have the potential to extend the range of electric vehicles to 1,000 miles or more. The potassium air battery is a very promising new member of the alkali metal air battery family. In theory, its weight energy density is more than three times that of the lithium ion battery. One of the key challenges encountered in designing a potassium-air battery is the need to select a suitable electrolyte. Such liquids can promote the transfer of particles between the anode and cathode of the battery to provide electricity. In general, when choosing an electrolyte, a trial-and-error method based on empirical rules is used, which is related to the characteristics of several electrolytes, and then a detailed (and time-consuming) test is performed on several candidate electrolytes to determine whether it meets the expected performance. Researchers at the University of Washington (St. Louis), led by Vijay Ramani, showed how to select an electrolyte for an alkaline metal air battery through a simple, easily measured parameter. Vijay Ramani is a Distinguished Professor of Roma B. and Raymond H. Wittcoff at the McKelvey School of Engineering Environment & Energy. Ramani's team studied the basic interaction between the salt and solvent in the electrolyte and showed how such effects affect the overall performance of the battery. They developed a new parameter, the "electrochemical" Tiller modulus (a measure of the ease of ion transport and reaction on the electrode surface). In addition, this study also applied the Nobel Prize winner Marcus-Hush's electron transfer theory for the first time to study the movement of ions in the electrolyte and their effects on the electrode surface. As solvent reorganization energy continues to increase, Tiller's modulus drops exponentially. The reorganization energy is a measure of the energy required to modify the solvated sphere. Therefore, solvent reorganization can be used to select a suitable electrolyte for high-performance metal-air batteries without other trial and error work. Shrihari Sankarasubramanian, a research scientist on the Ramani team, said: "In the beginning, we tried to better understand the effect of electrolytes on the redox reaction in metal-air battery systems, and finally showed how ions diffuse in the electrolyte and how these ions diffuse on the electrode surface How the reaction is, and such information is related to the energy required to break the solvated shell around the dissolved ions. Using a parameter to describe the relationship between solvation energy and ion transport and surface reaction kinetics is a breakthrough development that allows us for the rational development of new high-performance metal-air battery electrolyte. "(author: Yuqiu Yun) A snap ring (a portmanteau of "circle" and "clip"), also known as a C-clip, Rotor Clip, circlip or Jesus clip, is a type of fastener or retaining ring consisting of a semi-flexible metal ring with open ends which can be snapped into place, into a machined groove on a dowel pin or other part to permit rotation but to prevent lateral movement. There are two basic types: internal and external, referring to whether they are fitted into a bore or over a Shaft. Circlips are often used to secure pinned connections. Snap Ring,C-Clip Circlip,Single Spring Mechanical Seal,Multi Spring Mechanical Seal Ningbo FLK Technology Co., Ltd. , https://www.flk-global.com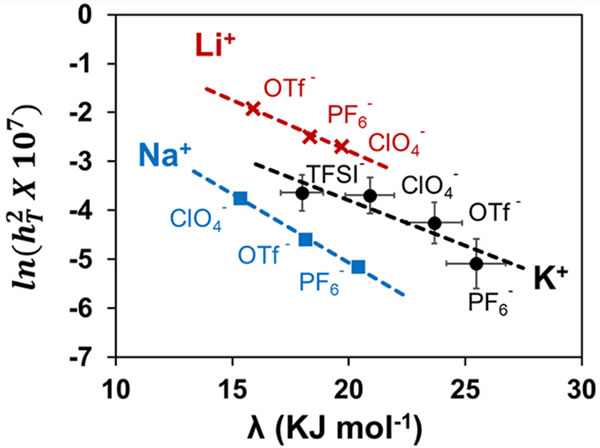
(Source: University of Washington)