We are a professional manufacturer of furniture chairs with dining chairs, office chairs, bar chairs, game chairs, folding chairs, etc.
product presentation:
Comfort and fashion game chair :
Ergonomic Office Chair :
American style modern high quality bar chair:
Modern simple dining chair :
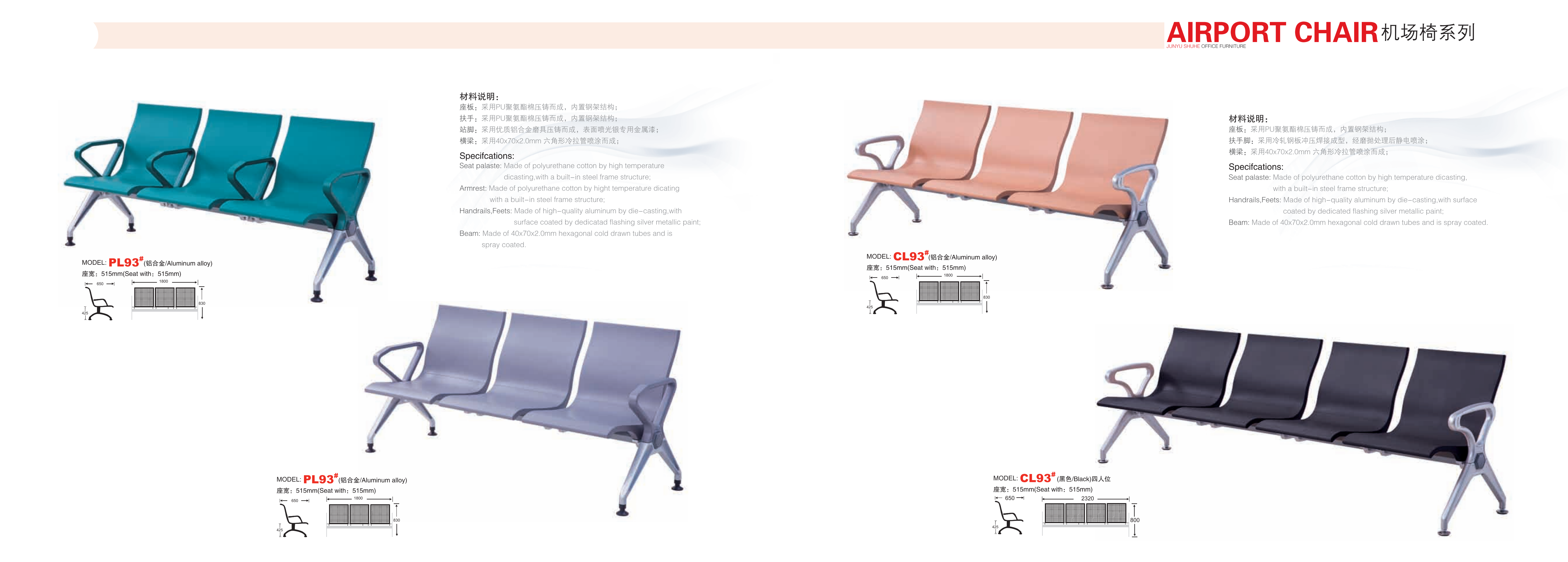
Airport Chair:
Bar Chair,Waiting Room Chair,Reliner Chair,Leisure Chair FOSHAN CITY LESHUN RONGHUI FURNITURE CO.LTD , https://www.ronghuifurniture.com
Discoloration is mainly caused by chemical changes between drugs or by light and air. Discoloration can affect the efficacy or even complete failure. Drugs that cause discoloration include alkalis, nitrites, and high-iron salts. For example, alkali drugs can cause aloe to produce green or red fluorescence, which can turn rhubarb into deep red; iodine and its preparations will be decolored with tannic acid. It is blue in combination with starch drugs; high-iron salts can turn tannin into blue.
Gas production means that gas is released during or after the preparation process, and the generated gas can be used to flush the bottle to cause the drug to be ejected, the efficacy will change, and even the container may explode. If sodium bicarbonate is combined with dilute hydrochloric acid, a neutralization reaction will occur to produce carbon dioxide gas.
When the precipitate is combined with two or more drug solutions, one or more insoluble solutes are produced. For example, calcium chloride and sodium hydrogen carbonate solution are combined to form poorly soluble calcium carbonate and precipitate; weak acid and alkali and salicylic acid Sodium solution, sodium sulfadiazine solution and the like are combined with hydrochloric acid to form water-insoluble salicylic acid and sulfadiazine to precipitate; for example, alkaloids in aqueous solution are treated with alkaline drugs, tannins, heavy metals, sulfonates and bromine The compound also produces precipitates and the like.
Hydrolysis Some drugs are prone to hydrolysis in aqueous solution and fail. For example, penicillin is easily hydrolyzed into penicillic acid in water, and its action is lost.
Combustion or explosion is caused by the combination of strong oxidant and strong reducing agent. For example, when potassium permanganate is mixed with glycerin, glycerin and nitric acid or ground together, it is prone to different combustion or explosion. The commonly used strong oxidant is potassium permanganate. Hydrogen peroxide, bleaching powder, potassium chloride, concentrated sulfuric acid, concentrated nitric acid, etc.; commonly used reducing agents are various organic substances, activated carbon, sulfide, iodide, phosphorus, glycerin, sucrose and the like.




Incompatibility of chemical drugs
Chemical incompatibility is the chemical reaction of certain drugs, which not only changes the traits of the drug, but also makes the drug less effective, invalid or toxic, and even causes burning or explosion. Chemical compatibility is common. The appearance phenomenon is discoloration, gas production, sedimentation, hydrolysis, combustion or explosion.
ã€Comment】 ã€Print this article】 ã€Close this page】 ã€Large, medium and small】